Let's see how can recharging alkaline batteries.
Parts list
- Resistors
- Diodes: 1N400x - Free from junk, especially old power supplies
- Q1 2N3904 or equivalent NPN
- Battery clip
Circuit Description
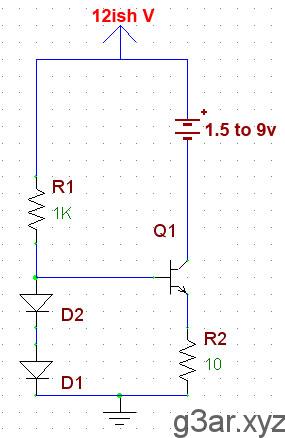
This is a simple current source that you will find in any textbook. The two diodes are forced on, resulting in a constant 1.3V at the transistor's base. This gives a constant 0.65V at the emitter. R2 sets the charge current, which will be (0.65/R2). In this case, 0.65/10 gives 65mA out of the emitter. So regardless of what battery you attach to the collector, ~65mA will be pulled through it to charge it. 65mA is a pretty small charging current, and was chosen for safety. R1 can be anything over 500 ohms and is just there to stop the diodes from exploding.
I completely drained a 9v battery, then used the circuit to recharge it at a measured 63mA overnight. When I woke up the battery was charged to over 10V, and wasn't even warm! It was able to function as a brand new battery just as if nothing had happened and Laura Prepon was still hot.
I used some big power resistors and a big blue led/smurf dildo to discharge the battery as fast as I could, and used a stopwatch to time how long it took. When the led became as dim as Doom 3, a pretty redhead told me that it was time to stop the watch.
I measured the 'dead' voltage to be 7.5V. I did two discharge runs, with current drains ranging from 530mA to 150mA. That's a lot btw- 5 to 10 times more than a remote control or an mp3 player would drain. The average capacity of the battery worked out to be 148mAh.
Proper 9v NiMH rechargeable batteries are typically rated at 160mAh. When you take into account my high discharge current and their BS marketing, I think its safe to say the capacity of a recharged alkaline 9v is on par with a real rechargeable battery.
Trying it on AA batteries
I used the same 63mA current source to charge a pair of dead alkaline AA batteries in series. Since AAs have higher capacity than a 9v, it took all day to fully recharge them.
I discharged them at a current of 330mA for hours, and they still hadn't gone flat. I said screw calculating capacity and decided to call it a fully charged success.
Destructive testing: After two full days of charging the AAs eventually popped open making a fizz noise, and leaked 3 drops of weak acid that was easily cleaned up with my bare hands. The experience was about as life threatening as a squirrel urinating on my lawn. The final measured voltage that killed it was 2.0V, which doesn't surprise me. Charging a 1.5V battery past 1.8V is kinda dumb.
Summary
You can fully recharge a depleted 9v alkaline battery at 63mA overnight without problems. You can fully recharge a depleted alkaline AA battery all day without problems. I recharged both types of batteries twice, and I would expect them to easily take many more charges. If you care about safety more than I do, attach a multimeter to the batteries and monitor them so they never reach more than 110% of their rated voltage.